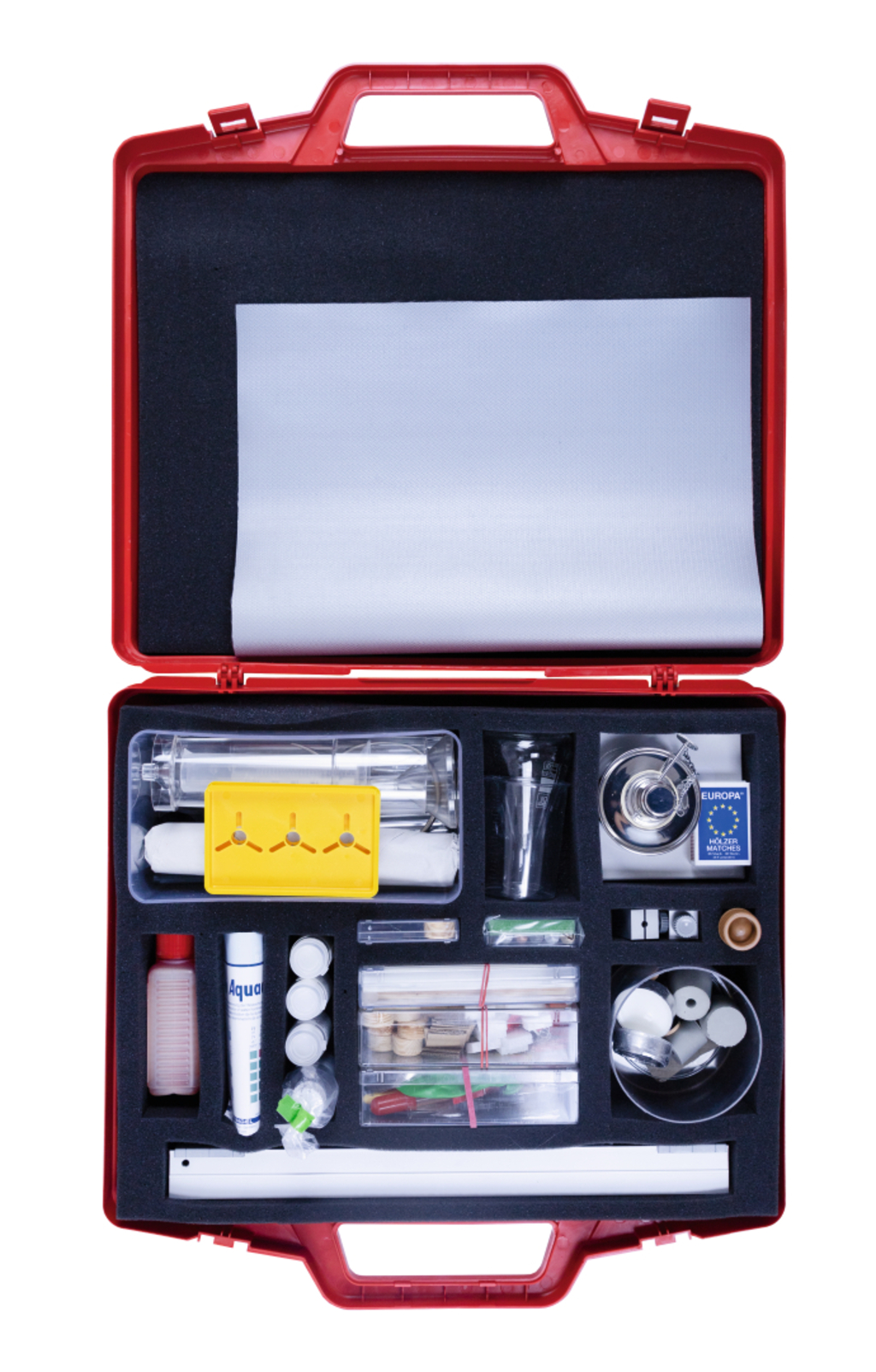
Students kit Materials in everyday life
This kit contains equipment and resources for scientific experiments covering multiple disciplines to study various substances. An initial section allows experiments to be carried out on basic material properties.
A second allows experimentation on mixtures of substances and separation of mixtures.
In addition, it is also possible to study changes of state between solid, liquid and gaseous substances and material changes due to chemical reactions.
Age 11-14
Materials for 1 work group or demonstration
Included in delivery:
- Experiment description with student worksheets
- Teacher's booklet with suggested solutions
Detailed instructions for 38 experiments:
Properties of materials
• Identifying and distinguishing materials
• Hardness and deformability of materials
• Density of materials
• Buoyancy of materials in liquids
• Thermal conductivity of– solid materials – liquid materials
• Heat resistance and ignition
• Magnetic behaviour
• Solubility
• Acidic and alkaline solutions
• Hard and soft water
• Consequences of water hardness
• Mineral salts in water
Mixtures of materials
• Mixing of solid materials
• Oil and water
• Mixing / Separation
• Separation by deposition (sedimentation)
• Separation by filtration, evaporation, vaporization/distillation, dissociation into constituent parts
• Production of drinking water from salt water
• Desalination of water
• Purification of dirty water by simple filtration
• Purification of dirty water by multi-layer filtration
• Magnetic separation for recycling scrap materials
Changing materials
• Changes in the state of liquids when warmed, gases when warmed, solid bodies when warmed
• Behaviour of bimetals when warmed
• Boiling of liquids
• States of aggregation of water
• Melting of materials
• Combustion and oxygen
• Release of gases
• Effect of gases
• Formation of rust
The same amount (same volume) of flour and sugar (or salt or semolina) is poured into the plastic pot with the help of the measuring cylinder. The substances are mixed well with the aid of the rod.
Subsequently, the experiment is repeated with different quantities of the respective substances.
The basis is the water and oil experiment. Here, the mixture in the test tube is sealed with a rubber stopper and shaken vigorously. The students now observe the behavior of the substances.
The students observe the liquids passing through a filter paper.
We will shortly provide you with a description of the experiment at this point.
We will shortly provide you with a description of the experiment at this point.
In this experiment, the students extract drinking water from salt water.
The pieces of material from the set of small materials are placed on the paper strip in random order. The paper strip is then slowly pulled under the bar magnet. The effects are observed.
The students feel and observe the different material samples from the suitcase.
The tip of the preparation needle is passed over the surfaces of the specimens one after the other with light pressure. It is observed whether traces remain and what the resulting traces look like
In this experiment, the students learn about the density of substances.
The students investigate the buoyancy of substances in liquids. And investigate the question of whether the immersion of the body in the liquid causes a change in the display.
Using an aluminum tube and a glass tube, students investigate the thermal conductivity of solids.
Water in a test tube is held over a spirit burner. The students observe whether the water heats up and whether the heat is also transferred to the test tube.
The students use combustion samples to investigate the heat resistance and ignition of the respective substances.
Using a bar magnet, the green side of the different materials is slowly approached from above. The students observe the behavior of the material samples.
With sugar and salt, students can observe the solubility of different substances in water.
It is observed whether a coloration of the water samples occurs due to the addition of the indicator solution. The colorations of the water samples are compared with a table and assigned accordingly.
The students test water with a water hardness test stick.
How does water with different water hardness behave? The students use a soap solution to find out.
With the help of nitrate/nitrite test strips, the students examine the mineral salts in the water.
First, water is poured into a test tube and then the oil. The students observe the behavior of the two liquids.
In this experiment, the students try to separate oil from water.
Water is poured onto a layer of sand or gravel. The students investigate the behavior in the still state and in the stirred state.
We will shortly provide you with a description of the experiment at this point.
We will shortly provide you with a description of the experiment at this point.
The contaminated water is poured into the filter bag in the funnel and closely observed as it passes through the filter paper. The condition of the water in the plastic pot is then assessed.
The dirty water is now slowly poured into the filter. It is observed how the dirty water passes through the two layers in the plastic pot. The condition of the water collected in the plastic pot is compared with the original dirty water. The water from the plastic pot is then poured back into the test tube and the entire process is repeated again.
The students observe the changes of state of liquids when they are heated.
We will shortly provide you with a description of the experiment at this point.
We will shortly provide you with a description of the experiment at this point.
The students observe the bimetal strip as it heats up.
The water whose boiling temperature is to be determined is poured into the small test tube. The small test tube is then immersed in the water in the large test tube. The thermometer is then placed in the small test tube.
The students observe the processes in the ice water and the temperature until the water boils.
The behavior of the substances is closely observed. Once the substance has melted, the burner is extinguished and the further behavior of the sample is observed.
The students observe the behavior of a candle flame when a plastic pot is placed over it.
The students observe the behavior of sherbet powder in water.
A burning tea light is placed in a plastic pot slightly filled with water. The sherbet powder mixture from the previous experiment is now slowly poured around the tea light into the surrounding water. The effects on the flame are observed.
In this experiment, the students observe how rust is formed through iron nails.
- 1 × °Glass with citric acid
- 4 × Nitrate/Nitrite test, 3 strips
- 4 × Water hardness test, 3 strips
- 1 × Holding clip on rod, 45 mm Ø (rod: 60x10 mm)
- 1 × Holding clip on rod, 25 mm Ø (rod: 60x10 mm)
- 2 × Tubular clip, 5x4 mm
- 1 × Foam insert, 430x320x20mm
- 1 × Carton for storage box, 481x425x107
- 1 × Funnel for the suction flask 47563, 60 mm ø
- 1 × Set of small material samples for magnets
- 1 × Dynamometer, 200 mN
- 1 × °Material patterns, set of 14 pcs.
- 1 × °Set of(5)metal cylinders
- 1 × Rail 360 mm with bores
- 1 × Set of combustion samples
- 1 × Foam insert for 22005 426x325x65 mm
- 1 × Vegetable oil, 30 ml
- 1 × Dissecting needle, 140 mmstraight with cap
- 1 × Heat resistant pad, 50x35 cm
- 1 ×
- 1 ×
- 2 ×
- 1 ×
- 1 ×
- 1 ×
- 1 ×
- 1 × °Glass with sugar
- 1 × °Glass with common salt (sodium chloride)
- 1 × Indicator solution, pH 4 - 10, 100 ml
- 1 × Spirit burner, metal
- 1 × Silicone tubing, 7/1,5 mmper m
- 1 × Test tubes, AR, 200 x 30 mm 10 pcs.
- 1 × Measuring cylinder, PP, 25 ml
- 1 × Rubber stopper 31/25 mm 1 hole 8 mm
- 1 × Rubber stopper 31/25 mm
- 1 × Rubber stopper 24/19 mm, 1 hole 8mm
- 1 × Glass tube, straight, 200 mm
- 1 × Glass tube, straight, 50 mm
- 1 × Filter paper, circular 90 mmØ, 100 pcs.
- 1 × Metal axis 110
- 2 × Watch glass, 80 mm
- 1 × Storage box, red 430x330x99 mm
- 1 × Magnet rod, AlNiCo red/ green, 100 x 10 mmØ
- 1 × Thermometer, red spirit -10+110°C:1°C
- 1 × Bimetallic strip
- 1 × Rubber balloons, 100 pcs.
- 2 × Clamp slider
- 1 × Retaining ring 75 mm Ø
- 2 × Bosshead with slit
- 1 × Stand rod, plastic 200x8 mm
- 1 × Aluminium tube, 200x8 mm
- 2 × Test tube stand, plastic
- 6 × Plastic test tube, 152 mm
- 1 × Matches (10 cases)
- 1 × Heat protection gauze
- 1 × Trough, plastic transp. 210x110x80 mm
- 1 × Metal spoon, 138 mm
- 1 × Plastic box 140/50/35 mm
- 1 × Coarse sieve
- 1 × Copper wire gauze
- 1 × Filter tube, transparent
- 1 × Plastic pot (old No.:90413)
- 1 × Colouring agent, red for lab use only
- 1 ×
- 2 ×
- 1 × Dropping pipette, plastic
- 1 × Beaker, plastic, 100 ml (graduated)
- 1 × Sewing thread, 100 m
- 2 ×